It can either donate three electrons or gain three electrons to complete its octet rather than losing its five electrons. As for the formation of a single covalent bond, one electron is shared by each atom
Atom
An atom is the smallest constituent unit of ordinary matter that has the properties of a chemical element. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small; typical sizes are around 100 picometers (1×10⁻¹⁰ m, a ten-milliont…
| Ligand | Electrons contributed (neutral counting) | Electrons contributed (ionic counting) |
|---|---|---|
| N | 3 | 6 |
| NR3 | 2 | 2 |
| CR2 | 2 | 4 |
| Ethylene | 2 | 2 |
How many electrons does nitrogen have?
Oct 12, 2021 · A nitrogen atom can fill its octet by sharing three electrons with another nitrogen atom, forming three covalent bonds, a so-called triple bond. How many electrons does two nitrogen share? Two nitrogen atoms will each share three electrons to form three covalent bonds and make a nitrogen molecule (N2).
Is nitrogen a donor or acceptor of electrons?
Mar 15, 2021 · Nitrogen has 5 valence electrons and is in a row with a maximum valence number of 8. It typically forms 3 bonds and has a lone pair (:NH 3) or makes 4 bonds with a positive charge (NH 4+ ). Nitrogen is one of the few elements that readily forms strong multiple bonds.
How many valence electrons does nitrogen gain or lose?
Nov 09, 2021 · It can either donate three electrons or gain three electrons to complete its octet rather than losing its five electrons. As for the formation of a single covalent bond, one electron is shared by each atom, thus nitrogen can share its three electrons to form 3 single covalent bonds.
How many bonds can a nitrogen atom have?
As Nitrogen has total 7 electrons.And in the outermost shell it has 5 electron.According to its electron arrangment nitrogen tends to share or accept the electron rather donating it. 235 views · Related answers
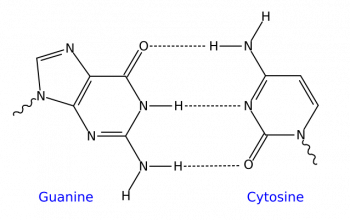
Does nitrogen donate or gain electrons?
Nitrogen, phosphorus, and arsenic can form ionic compounds by gaining three electrons, forming the nitride (N3-), phosphide (P3-) and arsenide (As3-) anions, but they more frequently form compounds through covalent bonding.
How many electrons does nitrogen lose?
Hint: The atomic number of nitrogen is 7 so the electronic configuration is $[He]2{s^2}2{p^3}$. Total 5 valence electrons are present in the outermost shell. The element gains or loses electrons to complete its octet of 8 electrons.
Does nitrogen have 3 or 5 valence electrons?
As you know, nitrogen exists as diatomic molecules, N2 . Each nitrogen molecule consists of two atoms of nitrogen that are bonded by a triple covalent bond. This is a direct consequence of the fact that each nitrogen atom has 5 valence electrons. Each atom can thus complete its octet by sharing three electrons.Jul 31, 2016
Can nitrogen have 10 valence electrons?
The total number of valence electrons is 5+6=11. Therefore, no matter how electrons are shared between the nitrogen and oxygen atoms, there is no way for nitrogen to have an octet.
How many bonds does nitrogen have?
As known, nitrogen could form 3 bonds based on octet rule, because it has 5 valence electrons. That means it needs 3 bonds.
How many electrons are in a metal nitrido complex?
An interstitial nitrogen can contribute 5 electrons, and the rest is provided by the group 9 and 10 metals which are electron-rich (typically, R h, I r ).
Nitrogen electron configuration via orbit
Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons of the atom revolve around the nucleus in a certain circular path. These circular paths are called orbit (shell). These orbits are expressed by n. [n = 1,2,3,4 .
Electron configuration of nitrogen atom through orbital
Atomic energy levels are subdivided into sub-energy levels. These sub-energy levels are called orbital. The sub energy levels are expressed by ‘l’. The value of ‘l’ is from 0 to (n – 1). The sub-energy levels are known as s, p, d, f.
How to write the orbital diagram for nitrogen (N)?
To create an orbital diagram of an atom, you first need to know Hund’s principle and Pauli’s exclusion principle. Hund’s principle is that electrons in different orbitals with the same energy would be positioned in such a way that they could be in the unpaired state of maximum number and the spin of the unpaired electrons will be one-way.
Nitrogen (N) excited state electron configuration
Atoms can jump from one orbital to another by excited state. This is called quantum jump. Ground state electron configuration of nitrogen is 1s 2 2s 2 2p 3. The p-orbital has three sub-orbitals. The sub-orbitals are p x, p y, and p z. Each sub-orbital can have a maximum of two electrons.
Electron configuration of nitrogen ion (N 3-)
After arranging the electrons, it is seen that the last shell of the nitrogen atom has five electrons. In this case, the valence electrons of nitrogen are five. The elements that have 5, 6, or 7 electrons in the last shell receive the electrons in the last shell during bond formation.
Determination of group and period through electron configuration
The last orbit of an element is the period of that element. The electron configuration of the nitrogen atom shows that the last orbit of the nitrogen atom is 2. So, the period of nitrogen is 2. On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element.
Determining the block of Nitrogen by electron configuration
The elements in the periodic table are divided into four blocks based on the electron configuration of the element. The block of elements is determined based on the electron configuration of the element. If the last electron enters the p-orbital after the electron configuration of the element, then that element is called the p-block element.
How many electrons, protons and neutrons does a nitrogen (N) atom have?
The nucleus is located in the center of the atom. Protons and neutrons are located in the nucleus. The atomic number of nitrogen is 7. The atomic number is the number of protons. That is, the number of protons in the nitrogen is seven. Electrons equal to protons are located in a circular shell outside the nucleus.
What are the valence electrons of nitrogen (N)?
The total number of electrons in the last shell after the electron configuration of nitrogen is called the valence electrons of nitrogen. The valence electron is the total number of electrons in the last orbit. The valence electrons determine the properties of the element and participate in the formation of bonds.
How do you calculate the number of valence electrons in a nitrogen (N) atom?
The valence electron has to be determined by following a few steps. The electron configuration is one of them. It is not possible to determine the valence electron without electron configuration. Knowing the electron configuration in the right way, it is very easy to determine the valence electrons of all the elements.
What is the valency of nitrogen (N)?
The ability of one atom of an element to join another atom during the formation of a molecule is called valency (valence). The number of unpaired electrons in the last orbit of an element is the valency of that element. As we know, the electron configuration of the nitrogen atom is normally 1s 2 2s 2 2p 3.
How many valence electrons does nitrogen ion (N 3-) have?
After arranging the electrons, it is seen that the last shell of the nitrogen atom has five electrons. In this case, the valence electrons of nitrogen are 5. We know the details about this.
Compound formation of nitrogen
Nitrogen participates in the formation of bonds through its valence electrons. This valence electron participates in the formation of bonds with atoms of other elements. Nitrogen atoms form bonds by sharing electrons with hydrogen atoms. The electron configuration of hydrogen shows that hydrogen has only an electron.

Popular Posts:
- 1. where to donate ald trophies in arkansas
- 2. where to donate used golf balls near me
- 3. how to donate blood plasma for money
- 4. where can i donate a old tv
- 5. where can i donate stuffed animals in pa
- 6. how to get people to donate money online
- 7. how ro donate servers to lineage os
- 8. how can i donate things to the poor in a foreign country
- 9. how to ask people to donate?
- 10. where to donate blood in nyc